
Published on nature synthesis (25 June 2024)
Author(s): Qiang Zhang, Charles B. Musgrave III, Yun Song, Jianjun Su, Libei Huang, Le Cheng, Geng Li, Yong Liu, Yinger Xin, Qiushi Hu, Ge Ye, Hanchen Shen, Xue Wang, Ben Zhong Tang, William A. Goddard III & Ruquan Ye
Abstract
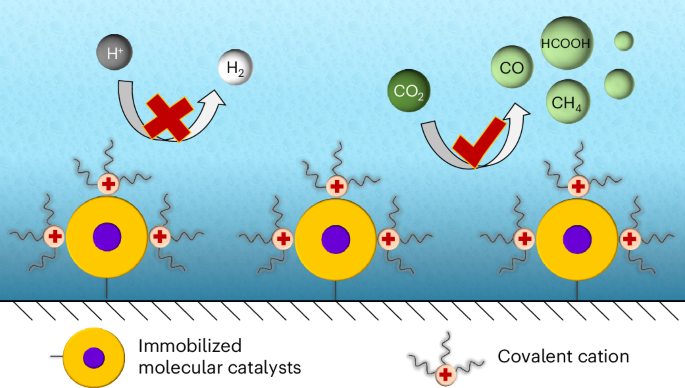
Molecular complexes are an important class of catalysts for the electrochemical carbon dioxide reduction reaction (CO2RR). However, selective CO2RR in strong acids remains challenging due to competition with the hydrogen evolution reaction. Peripheral functionalization is effective for tailoring the intrinsic activity of molecular catalysts, mostly attributed to the inductive effect or to stabilization of reaction intermediates. Here we report that peripheral functionalization of immobilized molecular complexes with quaternary ammonium groups can regulate the catalytic activity by tuning the mass distribution surrounding the active sites, enabling high-performance CO2RR in strong acids. The positively charged and hydrophobic alkylammonium groups affect the migration of water and hydronium in the double layer, while their immobilized configuration enables a stable cationic layer, inhibiting the hydrogen evolution reaction over extended potential windows. Dodecyl ammonium-functionalized cobalt phthalocyanine and tin porphyrin suppress the hydrogen Faradaic efficiency to <10% in pH ~0.5 media, while providing a single-pass conversion efficiency up to ~85%. The selectivity can be maintained at 90% even in Li+ solutions, which often exhibit poor proton shielding. Our study underscores the role of second-sphere structure for selective molecular electrochemistry.
Read more: https://www.nature.com/articles/s44160-024-00588-4