A research team co-led by chemists from City University of Hong Kong (CityU) recently discovered novel, highly effective anticancer agents with tridimensional structures, which have high anticancer activity, low toxicity and the ability to overcome drug resistance in cancer cells. The findings help provide a new direction for anticancer drug development.
Cancer has long been a devastating disease, which affects millions of people worldwide. Despite advances in treatment, current anticancer drugs often have limited effectiveness, lack of cancer selectivity, serious side effects and drug resistance in cancer cells.
“The structure of drugs greatly affects their anticancer performance,” explained Dr. Guangyu Zhu in the Department of Chemistry at CityU. “Most anticancer drugs have planar structures; developing new compounds with tridimensional structures may provide an opportunity to address the limitations of current anticancer drugs.”
In collaboration with researchers from The Hong Kong University of Science and Technology (HKUST), the team tested a new class of tridimensional and chiral compounds, which exhibit promising anticancer activity and present action mechanisms that are distinct from conventional anticancer drugs to overcome drug resistance.
The team first developed a new, highly efficient catalytic synthetic strategy to obtain a novel class of tridimensional and chiral tetraarylmethane compounds that presented better anticancer activity and lower toxicity than the clinical anticancer drug doxorubicin.
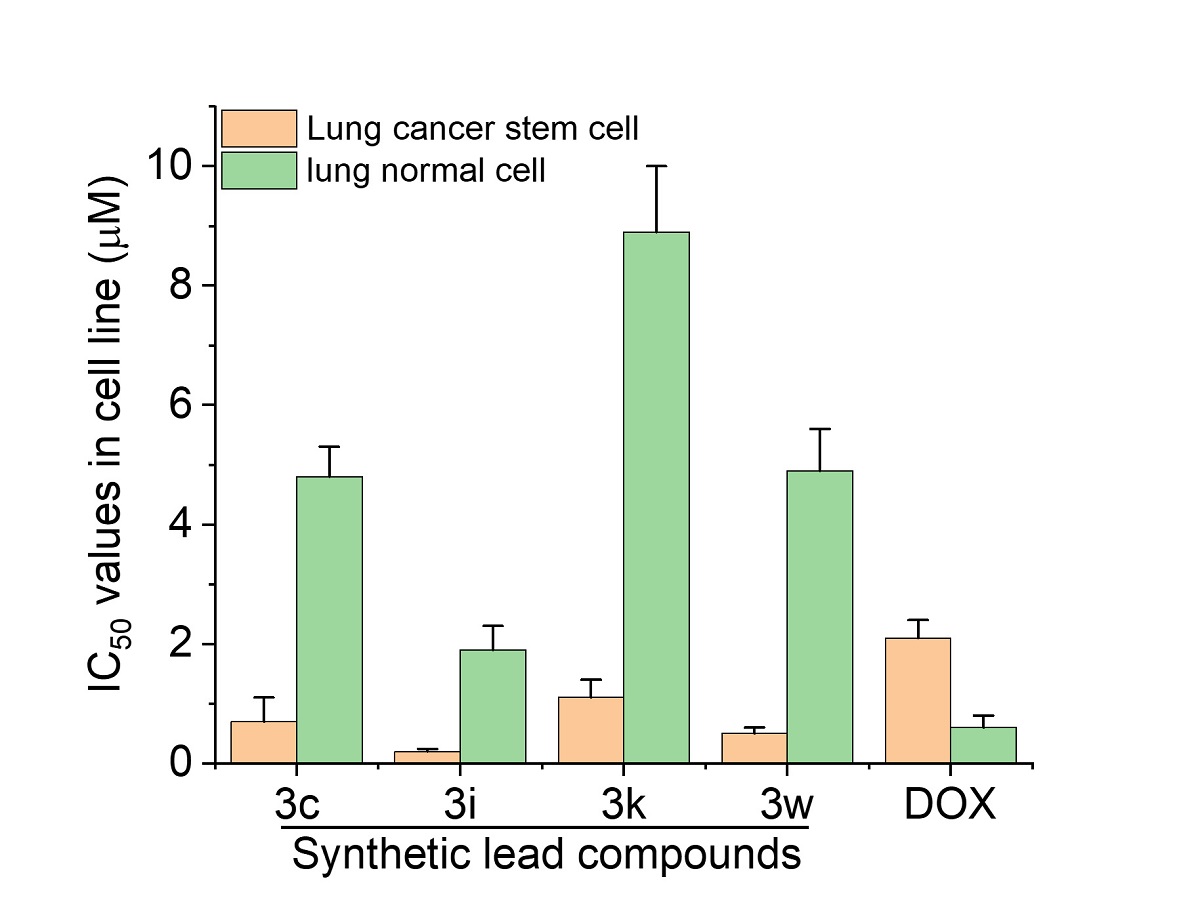
In their experiments, the research team tested the compounds with cancer cells in vitro, using doxorubicin as a control. They found that the tetraarylmethane compounds were more cytotoxic to cancer cells, including lung cancer stem cells (LCSCs), which are notorious for their drug resistance to clinical chemotherapeutic drugs, causing treatment failure. The compound also exhibited better cancer cell selectivity as it caused less harm to normal living cells, suggesting lower toxicity.
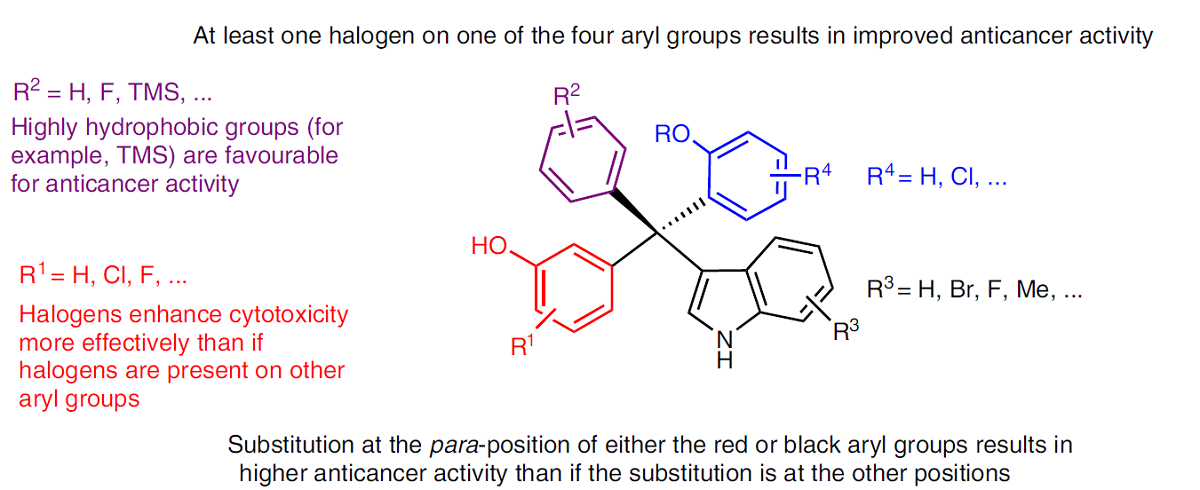
contribute to the anticancer activity of this type of compound are indicated. The presence of certain substituents, including halogen and hydroxyl groups, at certain positions in the tetraarylmethane compounds significantly improves cytotoxicity. TMS, trimethylsilyl; Me, methyl. Credit: ? Tan, X. et al.: https://www.nature.com/articles/s44160-022-00211-4
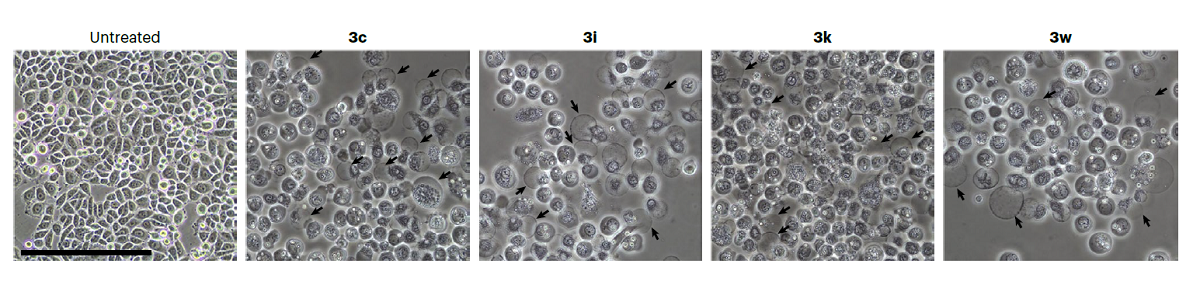
The team further analyzed the structure-activity relationship of synthesized compounds. They found that the presence of certain substituents, including halogen and hydroxyl groups, at certain positions of the tetraarylmethane compounds significantly improved their cytotoxicity to cancer cells. Upon treatment with the synthesized compound, some cancer cells started to die, as organelle swelling, cell membrane permeabilization, nuclear shrinkage and fragmentation were observed. This suggests that necrotic cell death might have been triggered by the tetraarylmethane compounds.
In the fight against cancer, the majority of anticancer drugs currently available rely on the activation of apoptotic pathways to eliminate cancer cells. However, a promising new avenue of research for reducing drug resistance lies in the development of novel anticancer agents that target alternative cell death pathways. In their experiments, the team found that these innovative compounds induced a different cell death pathway. This suggests that the compounds can bypass the resistance mechanisms generated by conventional drugs, making them highly desirable for further exploration in the field of cancer treatment.
“The satisfactory anticancer performance and unique mechanism make these compounds potential candidates for anticancer agents for further development,” said Dr. Zhu. The team plans to synthesize more compounds and conduct further experiments to evaluate their anticancer performance.
Their findings were published in the scientific journal Nature Synthesis under the title "Enantioselective synthesis of tetraarylmethanes through meta-hydroxyl-directed benzylic substitution".
The corresponding authors are Dr. Zhu and Professor Jianwei Sun from HKUST. The co-first authors are Dr. Xuefeng Tan from HKUST and Dr. Zhiqin Deng, former postdoc in Dr Zhu’s research group at CityU.
The research received financial support from the National Natural Science Foundation of China, the Science, Technology and Innovation Committee of Shenzhen Municipality, the Hong Kong Research Grants Council, and the Innovation and Technology Commission.
This research article originated from CityU Research Stories.