Detecting skin disorders based on tissue stiffness with a soft sensing device
By putting a piece of soft, strain-sensing sheet on the skin may be able to detect skin disorders non-invasively and in real-time very soon. A research team co-led by a scientist from City University of Hong Kong (CityU) has designed a simple electromechanical device that can be used for deep tissue pathology diagnosis, such as psoriasis, in an automated and non-invasive fashion. The findings will lay a foundation for future applications in the clinical evaluation of skin cancers and other dermatology diseases.
The research is co-led by Dr Yu Xinge, Assistant Professor from CityU’s Department of Biomedical Engineering, and scientists from and Northwestern University in the US. Their findings have been published in the science journal Nature Biomedical Engineering, titled “Miniaturized electromechanical devices for the characterization of the biomechanics of deep tissue”.
Electromechanical systems that enable precise, rapid measurements of the stiffness of soft tissues of the human body can provide useful clinical information for monitoring, diagnosing and treating various pathologies, particularly those of the skin. However, existing diagnostic evaluations, for example, magnetic resonance elastography, usually involve huge instruments at hospitals and trained practitioners. And the latest tissue stiffness-measuring technology based on sensing can only measure to superficial depths of upper skin, up to micrometre scale.
New device for real-time evaluations of deep tissue stiffness

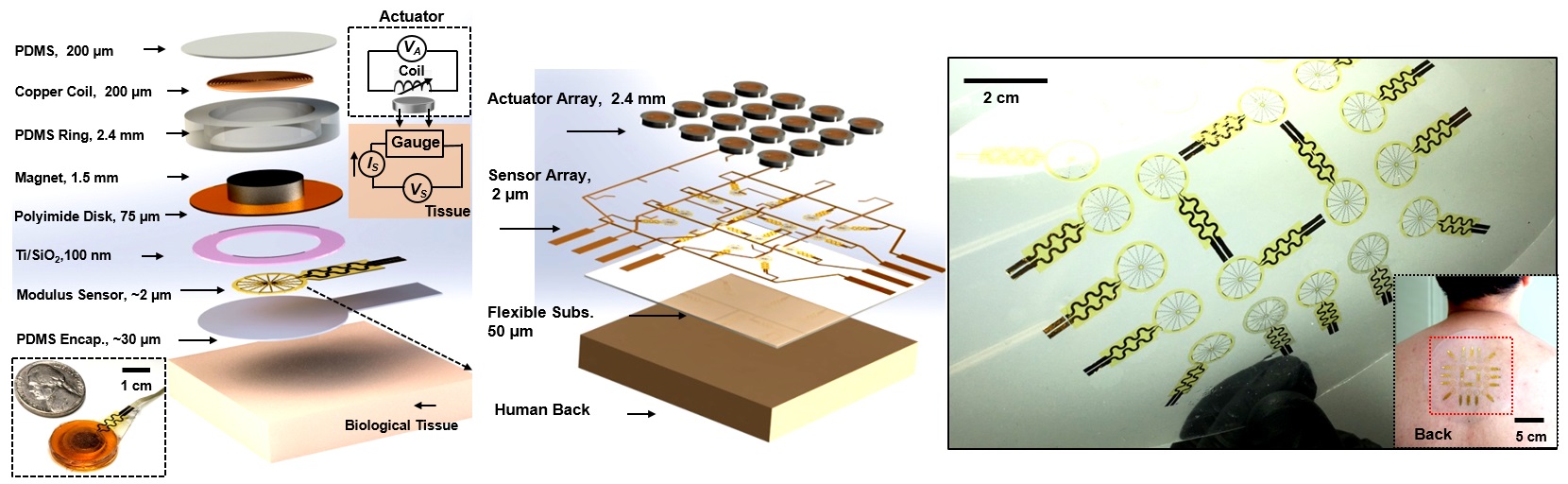
To address the issue, the research team designed a simple, miniature electromechanical device for high-precision, real-time evaluations of deep tissue stiffness. The team used a miniature electromagnetic system that integrates a vibratory actuator and a soft strain-sensing sheet to monitor in real-time the Young’s modulus, ie the tensile stiffness, of skin and other soft biological tissues at depths of approximately 1 to 8 mm, depending on the sensor designs.

The team evaluated the device’s performance with a range of synthetic and biological materials, such as hydrogels, pigskin and on various parts of human skin. “The lesions exhibited higher stiffness than those of the nearby skin, primarily due to differences in skin elasticity and hydration. These simple measurements have potential clinical significance in rapidly identifying and targeting skin lesions, with capabilities that complement those of recently reported methods for sensing mechanical properties at tissue surface (typically micrometre-scale),” explained Dr Yu. He pointed out that cancer tissue is typically stiffer or softer than normal tissue, and such difference can be used as diagnostic biomarker for a range of skin conditions, like skin cancer or tumours under the skin.
A simple structure of the electromechanical device
The electromechanical device’s thickness is only about 2.5 mm, and the contacting area is about 2 cm2. It operated well on both hair-bearing and hairless areas of the skin. Its working mechanism is adapted from the basis of a skin-integrated haptic interface technology for virtual/augmented reality developed by Dr Yu and the collaborators from Northwestern University before.

The device works like this: after applying an alternating current through the copper coil, the magnet vibrates and creates pressures onto the bottom surface of the sensor. This would direct deformations that extend to millimetre-scale depths of tissue, which leads to periodic variations in electrical resistance. Analyses of these responses by simultaneously measuring the voltage allow quantitative determination of the stiffness of the tissues. Each measurement could be done within one minute.
The team then conducted clinical studies on patients with skin disorders with their newly invented electromechanical device. The results indicated a potential for accurate targeting of lesions associated with psoriasis, showing the practical medical utility of the device. “The data produced can assist in diagnosis, treatment tracking and disease monitoring particularly for skin associated disorders, such as skin cancer, as well as in aspects of aesthetic dermatology and of the recovery from surface wounds,” said Dr Yu.
Dr Yu also pointed out that their device has the potential to be used for the evaluation of skin physical properties under various conditions such as ageing, hydration loss or associated dermatological disorders. “In the near future, we believe this technology will allow people to monitor their skin health status anytime with a simple wearable device,” said Dr Yu.
Dr Yu from CityU, together with Professor John A. Rogers, Professor Huang Yonggang and Dr Chang Jan-Kai from Northwestern University are the corresponding authors of the paper. Dr Song Enming, Senior Research Fellow in Dr Yu’s group, and Professor Xie Zhaoqian, a former Senior Research Fellow in Dr Yu’s group and now a Professor at the Dalian University of Technology, Professor Bai Wubin from University of North Carolina at Chapel Hill, and Dr Luan Haiwen from Northwestern University, are the first authors. Li Dengfeng, Yao Kuanming and Zhou Jingkun from CityU also participated in this research. Other researchers are from Northwestern Polytechnical University, The Pennsylvania State University, the University of Illinois at Urbana-Champaign, Sungkyunkwan University, Fudan University, and the Dalian University of Technology.
The research received funding support from including CityU, the National Natural Science Foundation of China, Fundamental Research Funds for the Central Universities, Ministry of Science and ICT of Korea, and National Science Foundation.
DOI number: 10.1038/s41551-021-00723-y