CityU researchers invents accurate rapid COVID-19 antibody level test
Vaccines have become the most important weapon in the fight against the COVID-19 pandemic, but antibody levels after vaccination decay quickly over time. Therefore, an accurate and affordable antibody rapid test is urgently needed to adjust the revaccination strategy. A research team led by City University of Hong Kong (CityU) recently invented an accurate rapid-testing device that can quantify and display the antibody level as a length of a visual bar, like a mercury thermometer, in as few as 20 minutes, enabling convenient mass screening or individual monitoring of immune protection against COVID-19.
The antibody level is a potent correlate of immune protection against virus infection, but it varies, depending on the vaccine type, and can decay rapidly. In the post-vaccine era, with the rise of the Omicron variants, it is critical to monitor antibody levels in a fast, cost-effective way, which can help provide an alert about the necessity for revaccination. However, existing antibody testing methods are either only for positive/negative results (like rapid antigen tests) or require equipment in professional laboratories or hospitals for quantification, which is expensive, time-consuming and impractical for routine tests.
A research team led by Dr Chen Ting-Hsuan, Associate Professor in the Department of Biomedical Engineering at CityU, recently developed a solution by inventing a new rapid antibody test that shows the level of antibodies readable by the naked eye. The findings were published in Science Advances under the title “Microfluidic particle dam for direct visualization of SARS-CoV-2 antibody levels in COVID-19 vaccinees”.
Displaying antibody levels in an easily readable way
“There have many attempts to miniaturize conventional immunoassay, such as enzyme-linked immunosorbent assay (ELISA) and electrochemistry, into a portable format. However, these miniaturized immunoassays all need a specific reader to pick up signals, big or small, which is expensive and error-prone if operated by untrained users,” explained Dr Chen. “In contrast, our unique device displays the antibody level directly on the chip, allowing users to see the antibody level by viewing the length of the bar on the chip, making it instrument-free and as easy to read as a thermometer, so it is more suitable for ordinary users.”
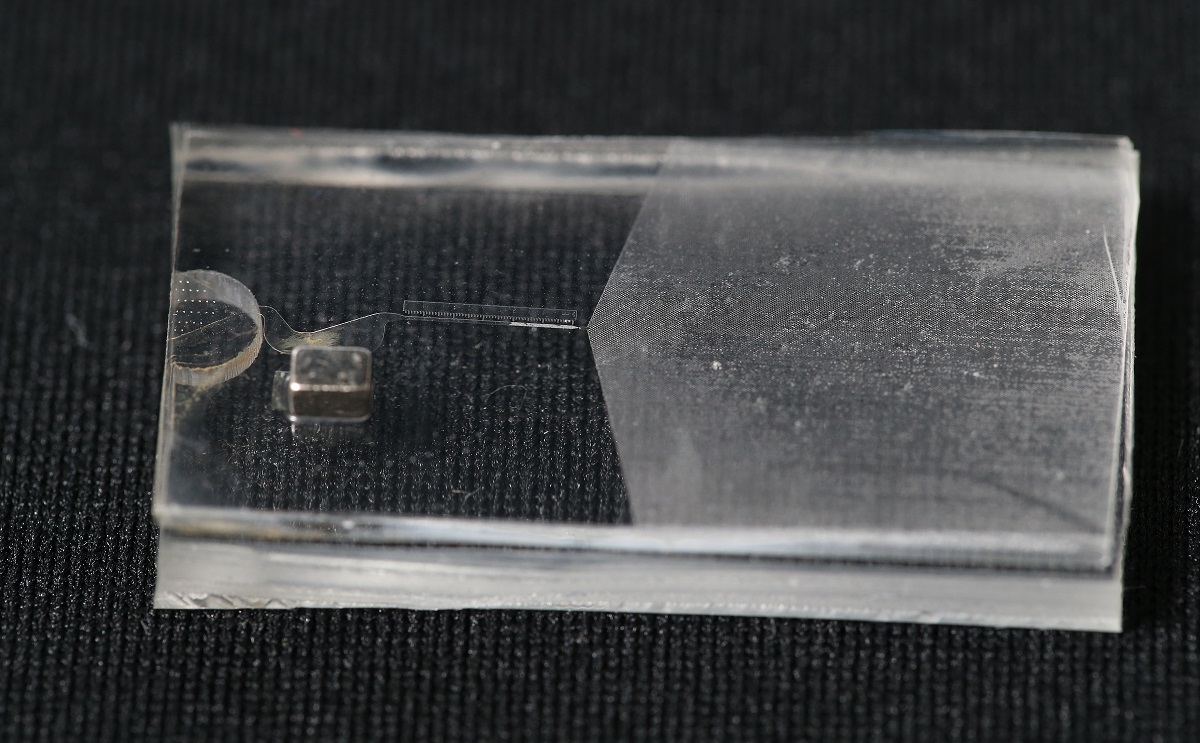
Photo credit: City University of Hong Kong
The rapid test developed by the team is a finger-sized microfluidic device that contains two types of specially designed microparticles – magnetic microparticles (MMPs) and polystyrene microparticles (PMPs). Both can bind to the antibody against SARS-CoV-2, which is the virus that causes COVID-19. If there are antibodies in the sample, they will bind with both the MMPs and PMPs, forming MMP-antibody-PMP joined microparticles.
Photo credit: Dr Chen Ting-Hsuan’s Research Team / City University of Hong Kong
Researchers just need to load the particle solution which was mixed with a few small drops of blood plasma collected from a user’s finger prick into the microfluidic chip. When the particle solution flows through the chip, a magnetic separator integrated into it removes the MMPs and the joined microparticles. Free PMPs that are not attached to MMPs continue to flow until they are trapped at a particle dam. As the PMPs accumulate, they form a visual bar whose length is inversely proportional to the antibody level in nanograms per millilitre. So it can quantify and visualize the antibody level as a thermometer measures temperature. (figure 1)
The research team tested the detection sensitivity of samples prepared by serial dilution of antibodies and found that the new device could detect antibodies as low as 10.75 ng/ml, which was much more sensitive than conventional rapid tests. More importantly, the detection results of the new device were accurate, as they closely agreed with the results obtained from the gold standard ELISA in a professional laboratory.
Procedure for rapid mode of detection. The magnetic microparticles are first mixed with undiluted serum/plasma for five minutes and shaken by hand to capture SARS-CoV-2 antibodies. After rinsing using a magnetic rack to remove the other interfering factors, polystyrene microparticles are added for another five minutes and shaken by hand. Next, the solution is dispensed into the loading chamber of the microfluidic device. After entering the micro-channel, within 10 minutes, an accumulation of polystyrene microparticles is formed that is visible to the naked eye. Credit: Wu., M. et al. / DOI: 10.1126/sciadv.abn6064
Highly sensitive and not easily interfered
The team then tested the detection performance of antibodies in undiluted serum to see whether the complex components within a biological specimen would interfere with the detection performance. The detection level can be adjusted to sensitive mode or rapid mode. Sensitive mode can detect antibodies in a concentration as low as 13.3 ng/ml within 70 minutes. Rapid mode, in contrast, takes only 20 minutes, but the lowest concentration detectable is 57.8 ng/ml.
The team recruited 91 volunteers in Hong Kong with no prior history of COVID-19, who received their second vaccine dose a few weeks before antibody testing. The results revealed that the antibody level reached 1080.8 ng/mL in those who received an mRNA vaccine (BioNTech), which was much greater than the antibody level of 30.2 ng/ml found in those who had received an inactivated vaccine (Sinovac). However, the antibody levels in both groups decayed significantly 45 days after the first measurement. Remarkably, the detection results were highly consistent with the results obtained from ELISA, suggesting the antibody test’s high accuracy comparable to the gold standard in professional laboratories.
“Our findings show that our microfluidic rapid test device allows low-cost, fast and accurate measurement of antibody levels by visual inspection, making it particularly suitable for time-saving mass-screening settings and individual use. The new rapid test could be used in local clinics or even testing booths at border entry control points in the future,” said Dr Chen. “This measurement may serve as an antibody-based ‘immunity passport’ for better evaluation of immune status to accelerate the economic recovery without adding to the medical burden on health care systems. This capability may lead to novel approaches to managing the pandemic in various countries, from zero-tolerance to co-existence with COVID-19.”
Dr Chen and his team are modifying the device to be more accessible and convenient for home use, such as making it capable of detecting antibodies in samples like saliva or nasal secretions.

Photo source: Dr Chen Ting-Hsuan / City University of Hong Kong
The first co-authors of the paper are Dr Wu Minghui, post-doc, and Miss Wu Siying, PhD student in the BME at CityU. Dr Chen is the corresponding author. This work was done in collaboration with Dr Iris Li Wai-sum, from The University of Hong Kong, and is supported mainly by the Innovation and Technology Fund.