Bimetallic alloy nanocatalyst boosts efficient ammonia production with potential for carbon-free energy
Ammonia (NH3) is regarded as a promising carbon-free energy carrier, but its energy-intensive production process still challenges global scientists. A research team led by City University of Hong Kong (CityU) recently engineered a bimetallic alloy as an ultrathin nanocatalyst that can deliver greatly improved electrochemical performance for generating ammonia from nitrate (NO3-), offering great potential for obtaining carbon-neutral fuel in the future.
Ammonia, which is commonly used in fertiliser, has recently attracted a lot of attention because it can provide a source of hydrogen for fuel cells, and it is easier to liquefy and transport than hydrogen. Owing to its huge demand, upcycling nitrate (NO3-) from ammonium fertiliser-polluted wastewater has emerged as an alternative for reproducing valuable ammonia and making agriculture more sustainable.
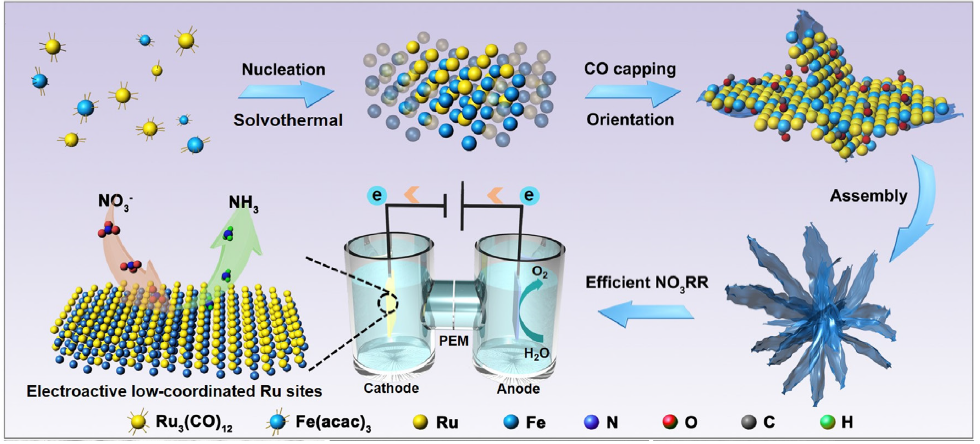
Currently, electrochemical nitrate reduction reaction (NO3RR) is regarded as a promising solution for ammonia synthesis. It comprises mainly deoxygenation and hydrogenation steps (i.e. NO3- + 9H+ + 8e- à NH3 + 3H2O) with metal-based electrocatalysts. “However, the undesired by-products and the competing hydrogen evolution reaction (HER) during NO3RR apparently hinders the yield rate of ammonia production,” said Professor Fan Zhanxi, of the Department of Chemistry at CityU, who led the study.
Instead of modulating the electrocatalysts’ size or dimension, as other previous research did, Professor Fan’s team focused on improving the active sites, where substrate molecules bind and catalysis takes place on the surface of the electrocatalysts.
“Ruthenium (Ru) is an emerging material as an electrocatalyst for NO3RR, but it also has the problem of favouring HER, which leads to its active sites being highly occupied by undesired active hydrogen, leaving insufficient area for nitrate reduction into ammonia,” explained Professor Fan.
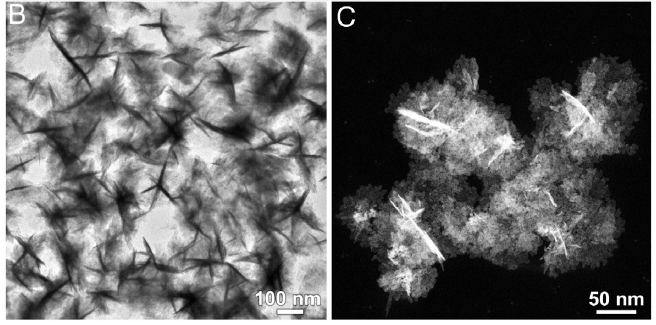
To overcome the challenges, the team introduced another metal – iron (Fe) – to modulate the atomic coordination environment of the active sites. By changing the coordination environment of the Ru sites, the electronic structures and surface properties of Ru and hence their catalytic activity for producing ammonia are optimised. To further enhance the electrocatalyst performance, the team developed a one-pot synthesis approach for making ultrathin nanosheets that are assembled as a flowerlike structure – called RuFe nanoflowers.
This novel bimetallic alloy made-electrocatalyst possesses a highly stable electronic structure due to the complementary orbitals that reach efficient electron transfer and robust valence states, which also suppresses the competitive HER and lowers the energy barriers for NO3RR. Moreover, the electrochemically active surface sites of the RuFe nanoflowers measured 267.5 cm2, much larger than the 105 cm2 for Ru-nanosheets for the reactions to take place.
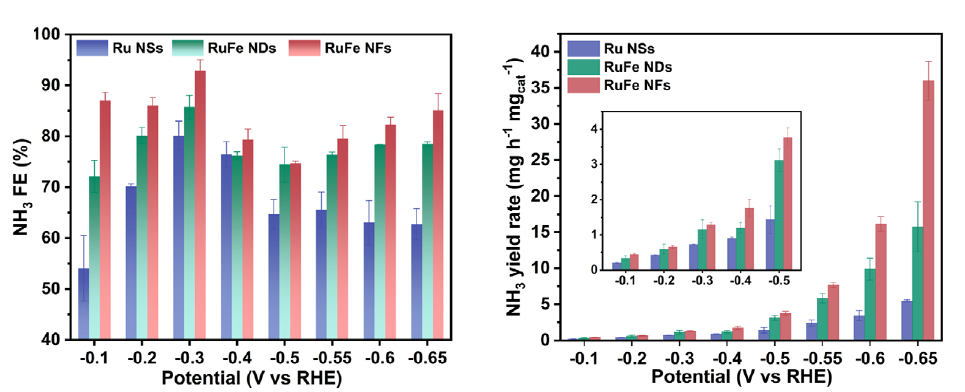
Remarkably, RuFe nanoflowers demonstrated much better electrochemical performance, with an outstanding charge transfer efficiency, known as faradaic efficiency (FE), of 92.9% and a yield rate of 38.68 mg h?1 mgcat?1 at ?0.30 and ?0.65 V for ammonia production, which is almost 6.9 times that of sole Ru-nanosheets.
“This research indicates great potential for RuFe nanoflowers in next-generation electrochemical energy systems,” said Professor Fan. “We believe this work can stimulate follow-up studies on modulating the atomic coordination environment of active sites in metal-based catalysts for ammonia production, further promoting a sustainable nitrogen cycle to achieve carbon-free energy in the future.”
The findings were published in the scientific journal The Proceedings of the National Academy of Sciences (PNAS) under the title “Atomic coordination environment engineering of bimetallic alloy nanostructures for efficient ammonia electrosynthesis from nitrate”.

The study’s co-first authors are Mr Wang Yunhao, Mr Zhou Jingwen and Mr Xiong Yuecheng, all of whom are PhD students in the Department of Chemistry at CityU, and Dr Sun Mingzi, from The Hong Kong Polytechnic University (PolyU). The corresponding authors are Professor Fan, Professor Huang Bolong, from PolyU, Professor Gu Lin, from Tsinghua University, and Professor Chu Shengqi, from the Institute of High Energy Physics at the Chinese Academy of Sciences (CAS).
This research is supported by the National Natural Science Foundation of China, the Hong Kong Research Grants Council, the Innovation and Technology Commission, and CityU.