Revolutionizing stable and efficient catalysts with turing structures for hydrogen production
Hydrogen energy has emerged as a promising alternative to fossil fuels, offering a clean and sustainable energy source. However, the development of low-cost and efficient catalysts for hydrogen evolution reaction remains a crucial challenge. A research team led by scientists from City University of Hong Kong (CityU) has recently developed a novel strategy to engineer stable and efficient ultrathin nanosheet catalysts by forming Turing structures with multiple nanotwin crystals. This innovative discovery paves the way for enhanced catalyst performance for green hydrogen production.
Producing hydrogen through the process of water electrolysis with net-zero carbon emissions is one of the clean hydrogen production processes. While low-dimensional nanomaterials with controllable defects or strain modifications have emerged as active electrocatalysts for hydrogen-energy conversion and utilization, the insufficient stability in these materials due to spontaneous structural degradation and strain relaxation leads to their catalytic performance degradation.
To addressing this issue, a research team led by Professor Lu Jian, Dean of the College of Engineering at CityU and Director of Hong Kong Branch of National Precious Metal Material Engineering Research Center, has recently developed a pioneering Turing structuring strategy which not only activates but also stabilizes catalysts through the introduction of high-density nanotwin crystals. This approach effectively resolves the instability problem associated with low-dimensional materials in catalytic systems, enabling efficient and long-lasting hydrogen production.
Credit: Gu J. et al, source: https://www.nature.com/articles/s41467-023-40972-w
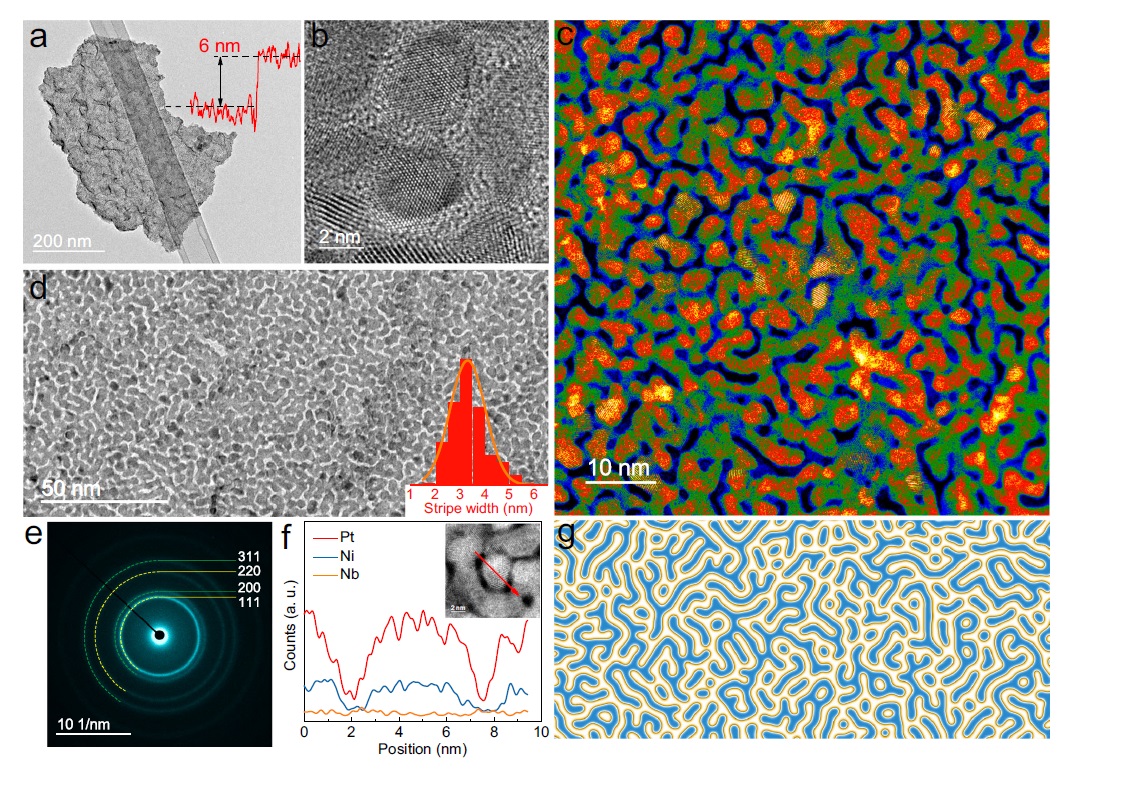
Turing patterns, known as spatiotemporal stationary patterns, are widely observed in biological and chemical systems, such as the regular surface colouring on sea-shells. The mechanism of these pattern formations is related to the reaction-diffusion theory proposed by Alan Turing, a famous English mathematician regarded as one of the fathers of modern computing, in which the activator with a smaller diffusion coefficient induces local preferential growth.
“In previous research, the fabrication of low-dimensional materials has mainly focused on structural controls for functional purposes, with few considerations on spatiotemporal controls,” Professor Lu explained the background of this research. “However, the Turing patterns in nanomaterials may be achieved by the anisotropic growth of nanograins of the materials. Such broken lattice symmetry has crucial crystallographic implications for the growth of speci?c con?gurations, such as two-dimensional (2D) materials with twinning and intrinsic broken symmetry. So we wanted to explore the application of Turing theory on nanocatalyst growth and the relations with crystallographic defects.”
In this research, the team used two-step approach to create superthin platinum-nickel-niobium (PtNiNb) nanosheets with strips topologically resemble Turing patterns. These Turing structures on nanosheets were formed through the constrained orientation attachment of nanograins, resulting in an intrinsically stable, high-density nanotwin network which acted as structural stabilizers which prevented spontaneous structural degradation and strain relaxation.
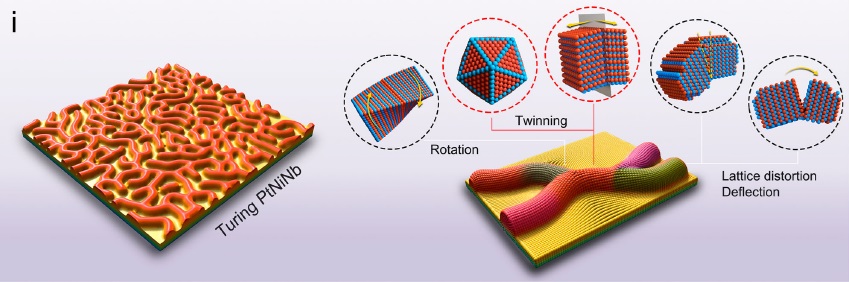
Moreover, the Turing patterns generated lattice straining effects which reduce the energy barrier of water dissociation and optimize the hydrogen adsorption free energy for hydrogen evolution reaction, enhancing the activity of the catalysts and providing exceptional stability. The surface of the nano-scale Turing structure exhibits a large number of twins interfaces, also rendering it an exceptionally well-suited materials for interface-dominated applications, particularly electrochemical catalysis.

Credit: Gu J. et al, source: https://www.nature.com/articles/s41467-023-40972-w
In the experiments, the researchers demonstrated the potential of the newly invented Turing PtNiNb nano-catalyst as a stable hydrogen evolution catalyst with superb efficiency. It achieved 23.5 and 3.1 times increase in mass activity and stability index, respectively, compared with commercial 20% Pt/C. The Turing PtNiNb-based anion-exchange-membrane water electrolyser with a low platinum (Pt) mass loading of 0.05 mg cm?2 was also extremely reliable, as it could achieve 500 hours of stability at 1,000 mAcm?2.
“Our key findings provide valuable insights into the activation and stabilization of catalytic materials with low dimensions. It presents a fresh paradigm for enhancing catalyst performance,” said Professor Lu. “The Turing structure optimization strategy not only addresses the issue of stability degradation in low-dimensional materials but also serves as a versatile material optimization approach applicable to other alloying and catalytic systems, ultimately enhancing catalytic performance.”

Photo credit: City University of Hong Kong
The findings are published in Nature Communications, titled “Turing structuring with multiple nanotwins to engineer efficient and stable catalysts for hydrogen evolution reaction”.
The first authors are –Dr Gu Jialun and Miss Li Lanxi from CityU. The corresponding author is Professor Lu. Other collaborators from CityU include Dr Xie Youneng, Dr Chen Bo, Dr Wang Yanju, Mr Zhong Jing and Dr Shen Junda.
The research was supported by CityU, the General Research Fund Scheme, the Innovation and Technology Commission of HKSAR, the National Key R&D Program of China, the Guangdong Provincial Department of Science and Technology and others.