Curved carbon nanotubes enhance electrocatalysts for carbon neutrality
Electrocatalysis plays a vital role in developing clean energy, greenhouse gas removal and energy storage technologies. A study co-led by City University of Hong Kong (CityU) researchers found that single-walled carbon nanotubes are excellent substrates for enhancing greenhouse gas conversion through molecular curvature. By using these nanotubes as support to induce strain on an electrocatalyst, the efficiency of carbon dioxide reduction to methanol can be significantly improved. This breakthrough opens avenues for developing curved molecular electrocatalysts to efficiently convert carbon dioxide (CO2), one of the key greenhouse gases, into useful chemicals and fuels, thus reducing carbon emission.
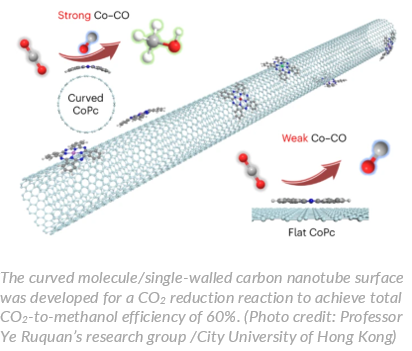
Many molecular complexes, like cobalt phthalocyanine (CoPc), are efficient catalysts for CO2 reduction reaction (CO2RR). However, they primarily reduce CO2 to poisonous carbon monoxide (CO), without further generating a substantial amount of useful products, like methanol. “Therefore, we want to explore the potential of CoPc beyond CO production,” said Professor Ye Ruquan, of the Department of Chemistry at City University of Hong Kong (CityU), who led the research.
At the same time, strain is known to affect the properties of two-dimensional materials which are often at the scale of nanometres (nm). “The use of curved substrates, or supports, to induce local strain is well established for modulating the properties of conventional layered materials,” explained Professor Ye. “But rational control of the strain of planar molecules is challenging due to their ultra-small size. And how the strain affects molecular properties remains poorly understood.”
Together with his collaborators, Professor Ye led a research team to investigate the reactivity for molecular CoPc catalysts at the nanometre scale by adopting support-induced strain engineering. They successfully introduced controlled-strain into sub-2 nm molecules of the catalyst by using single-walled carbon nanotubes as the support. The curvature of the nanotubes due to the molecular interactions induces strain on the catalytic molecules, resulting in bending. Using carbon nanotubes substrates with different diameters allows them to tune the bending angle of CoPc molecules ranging from 96° (for 1-nm-diameter carbon nanotubes) to 1.5° (for 100-nm-diameter carbon nanotubes).
Compared with traditional planar molecules, the curved molecules exhibited improved electrocatalytic performance. They showed higher selectivity for CO2 reduction, favouring the production of methanol over carbon monoxide.
In a tandem-flow electrolyser with monodispersed CoPc on single-walled carbon nanotubes for CO2 reduction, the team achieved a methanol partial current density of more than 90 mA cm?2 with over 60% selectivity, meaning that the total CO2-to-methanol efficiency is 60%. This is a significant improvement over existing methods.
Their analysis based on theoretical calculations confirmed that the curved CoPc on the single-walled carbon nanotubes enhanced CO binding, enabling the consequent reduction of carbon monoxide. In contrast, wide multi-walled carbon nanotubes favour the release of CO.
“Our findings show that carbon nanotubes are exceptional support materials for catalysts like CoPc. The large specific surface areas of carbon nanotubes readily disperse nanoparticles, avoiding agglomeration, and their high electronic conductivity make them promising for electrochemical applications,” said Professor Ye.
“More importantly, we showed that inducing molecular distortion through single-walled carbon nanotubes provides a strategy for designing high-performance molecular electrocatalysts. This advancement holds promise for achieving carbon neutrality, as it can store CO2 and renewable electricity as chemical energy,” he concluded.
The findings were published in Nature Catalysis under the title “Strain enhances the activity of molecular electrocatalysts via carbon nanotube supports”.
The first authors are Dr Su Jianjun, a postdoct in the Department of Chemistry, and Charles B. Musgrave III, from the California Institute of Technology (Caltech). The corresponding authors are Professor Ye and Professor William A. Goddard III, from Caltech.
The research was supported by various organisations, including CityU, the Guangdong Basic and Applied Basic Research Fund, the Hong Kong Research Grants Council and the State Key Laboratory of Marine Pollution.
