Efficient bifunctional catalyst enables high-performance zinc-nitrate / ethanol batteries for sustainable energy storage
Zinc-nitrate batteries are a primary non-rechargeable energy storage system that utilizes the redox potential difference between zinc and nitrate ions to store and release electrical energy. A research team co-led by chemists from City University of Hong Kong (CityU) have developed a high-performance rechargeable zinc-nitrate/ethanol battery by introducing an innovative catalyst. They successfully designed and synthesized an efficient tetraphenylporphyrin (tpp) modified heterophase rhodium-copper alloy metallene (RhCu M-tpp). This bifunctional catalyst exhibits remarkable capabilities in both the electrocatalytic nitrate reduction reaction (NO3RR) and ethanol oxidation reaction (EOR) in a neutral medium, overcoming the monofunctional limitations of traditional metal-based solid catalysts and providing a valuable reference for the design of sustainable energy storage in the future.
“This study highlights the significance of molecule-metal relay catalysis to efficient NH3 electrosynthesis in NO3RR and offers a multifunctional battery prototype that shows the benefits of metal-based hybrid electrochemical systems on high-performance, sustainable energy storage and conversion,” said Professor Fan Zhanxi, Assistant Professor in the Department of Chemistry at CityU, who led the study, highlighting the significance of the findings.
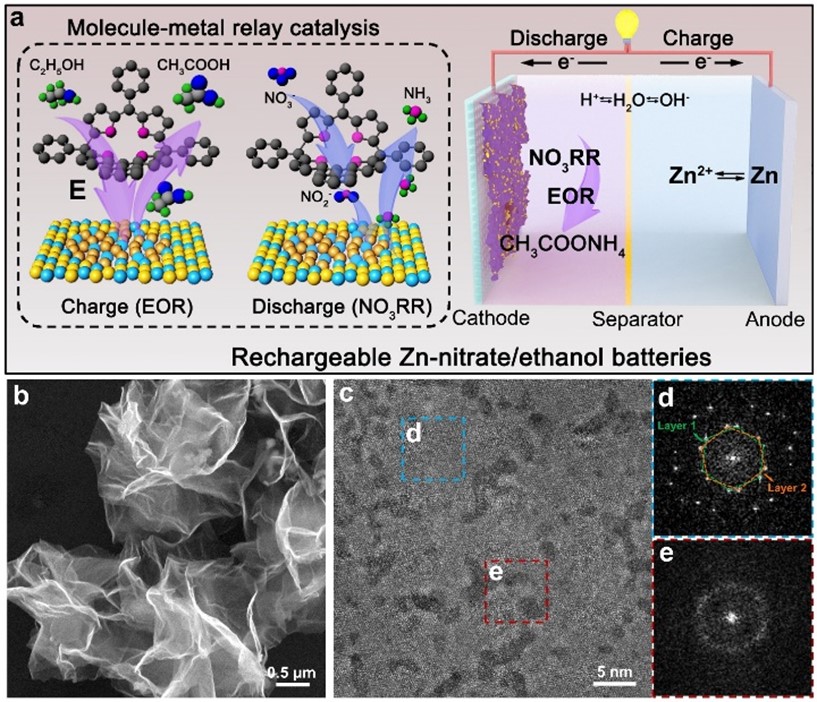
To elaborate on the uniqueness of the findings, Prof Fan explained that the as-obtained RhCu M-tpp overcomes the challenge of traditional Cu-based catalysts requiring quite negative potential to efficiently convert nitrate to ammonia when conducting NO3RR in a neutral medium. Moreover, based on the superior bifunctionality of as-prepared RhCu M-tpp for both NO3RR and EOR, a rechargeable Zn-nitrate/ethanol battery was successfully constructed to address the poor rechargeability of traditional zinc-nitrate galvanic cells.
Additionally, a molecule-metal relay catalysis mechanism was unraveled in this work, whereby nitrate is firstly reduced to nitrite on tpp, and then the as-generated nitrite is converted into ammonia on metallic sites. This verified the feasibility of molecular surface modification for improving the electrochemical performance of nanometals for NO3RR.
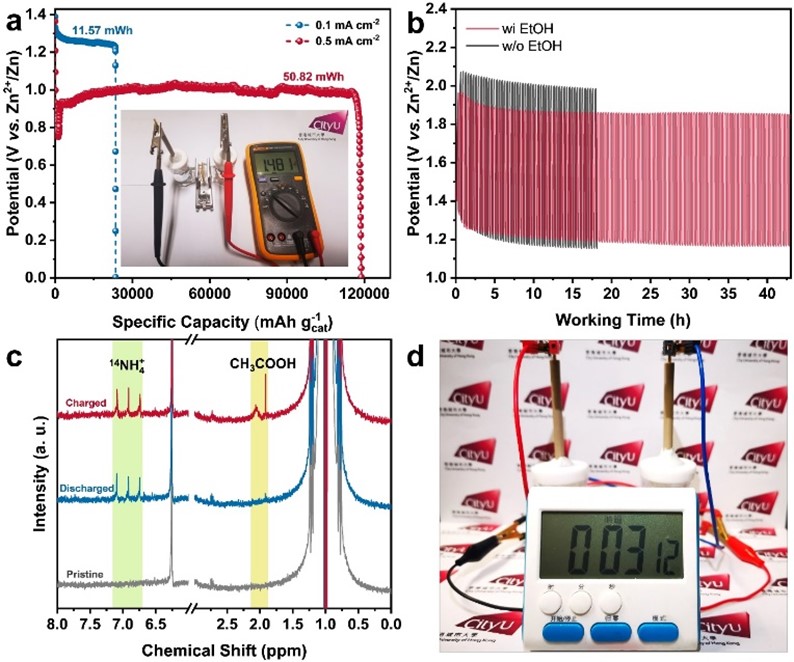
The activity of cathode catalysts is crucial for the performance of zinc-nitrate batteries. However, currently, used copper-based catalysts have limitations. They require highly negative applied potential and have weak proton adsorption, resulting in low current density and ammonia yield. Additionally, these catalysts are not suitable for electrocatalytic oxygen evolution reaction (OER), leading to non-rechargeable batteries and poor cycling life.
To address these issues, the research team developed ultrathin bimetallic RhCu metallenes to reduce the energy barrier for copper. After many attempts, they discovered that modifying the surface of RhCu metallenes with a small molecule, called tpp, significantly improved the efficiency of nitrate conversion to ammonia without compromising the performance of metallic substrates in ethanol oxidation. This breakthrough can thus enhance the overall performance of zinc-nitrate batteries.
The research findings offer an effective solution for constructing high-performance, zinc-based hybrid energy systems and provide valuable insights for future catalyst design of multifunctional and environmentally friendly devices.
The findings were published in the scientific journal PNAS, titled “Constructing molecule-metal relay catalysis over heterophase metallene for high-performance rechargeable zinc-nitrate/ethanol batteries”.

For enquiry, please contact Professor Fan Zhanxi from the Department of Chemistry in CityU, by email at zhanxi.fan@cityu.edu.hk.