Discovery of a protein that promotes cancer metastasis

A research team led by City University of Hong Kong (CityU) has discovered a novel protein, Lysyl hydroxylase 1 (LH1), which is a key factor in promoting cancer cell migration and metastasis in liver cancer (hepatocellular carcinoma, HCC) and pancreatic cancer (pancreatic ductal adenocarcinoma, PDAC). They also found that a high LH1 level is associated with poor prognosis (the development of disease and long-term survival) of HCC and PDAC patients. The team expects the research findings to provide a new potential treatment target for cancer therapy.
Cancer metastasis is a major cause of cancer-related death, and the migration of cancer cells through increasingly stiff solid tumours is a common feature of HCC and PDAC metastasis, but the mobility of cells in the tumour microenvironment remains poorly understood. “We aim to study the molecular mechanism of cancer cell migration in the confined microenvironment and to identify novel genes and proteins related to the process,” said Professor Michael Yang Mengsu, Vice-President (Research and Technology) and Yeung Kin Man Chair Professor of Biomedical Sciences at CityU, who led a multi-institution team to conduct the research.
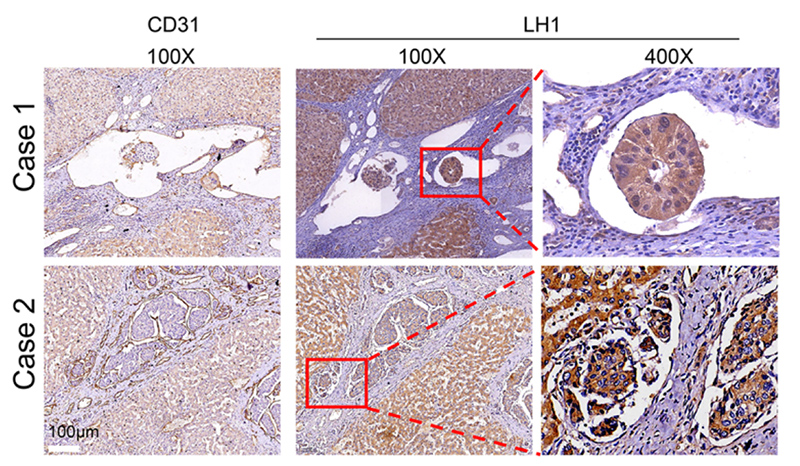
The research team discovered that LH1 enhances the migration capability, including speed and invasion capacity of HCC and PDAC cells in confined space through binding and stabilising Septin2 (SEPT2), a protein that plays an essential role to ready the cells for the high mechanical demands of migration, thus promoting the metastasis of HCC and PDAC cells. They also discovered that high LH1 expression is correlated with poor prognosis for both HCC and PDAC patients. The findings were published on 31 January 2023 in Molecular Cancer, a leading peer-reviewed journal on cancer-related research from a molecular perspective.

The research work was carried out mainly by CityU PhD student Eileen Yang Zihan and Dr Zhou Zhihang of the Second Affiliated Hospital of Chongqing Medical University. The multi-institution research team consists of researchers from the Tung Biomedical Sciences Centre of CityU, the Second Affiliated Hospital of Chongqing Medical University, the Precision Medical Technology Centre of CityU Futian Research Institute, and the Hong Kong Polytechnic University.
“The main challenge in this research is recreating the complex cancer microenvironment, but the team successfully developed a series of multidimensional 2D and 3D in vitro and in vivo models to comprehensively study the cancer cell migration process in confined space,” explained Professor Yang. “The findings are expected to provide a potential new target for cancer diagnosis and drug development.”