New in vivo priming strategy to train stem cells can enhance cardiac repair effectiveness
Human stem cells have been regarded as one of the promising cell sources for cardiac regeneration therapy. But their clinical use is hampered due to the poor performance after transplantation into failing hearts. Recently a stem cell biologist from City University of Hong Kong (CityU), together with his collaborators, has developed a novel strategy, called in vivo priming, to “train” the stem cells to stay strong after implantation to the damaged heart via the 3D-printed bandage-like patch. The positive results of the study show that an in vivo priming strategy can be an effective means to enhance cardiac repair.
Dr Ban Kiwon, Assistant Professor of CityU’s Department of Biomedical Sciences, collaborated with cardiologist and experts in 3D printing from South Korea in achieving this breakthrough. Their findings were published in the scientific journal Science Advances, titled “In vivo priming of human mesenchymal stem cells with hepatocyte growth factor-engineered mesenchymal stem cells promotes therapeutic potential for cardiac repair”.
Harsh environment in failing hearts hinders stem cell survival
One of the proposed approaches to treat myocardial infarction, commonly known as heart attack, with regeneration therapy is to inject the human stem cells directly into the failing hearts. In particular, human mesenchymal stem cells (hMSCs) have been considered as a competitive agent for clinical uses for their proven safety and significant paracrine effects supporting new blood vessel formation and inhibiting cell death. However, “the clinical trial results are disappointing as the micro-environment of a failing heart is very harsh for the injected hMSCs to stay alive,” said Dr Ban.
Therefore researchers have been exploring ways to increase the survival rate of hMSCs in failing hearts. “Priming, or called preconditioning, is a common strategy to empower the cells. The cells are educated through certain stimulations, and when they are relocated to tough environments, they are much stronger against bad condition and they will know how to react because of their previous experiences,” explained Dr Ban.
Conventionally, priming is performed in vitro (outside a living organism) before the cells are transplanted into the heart. “But the effects of priming done in this way usually last for two or three days only. To extend the duration of the priming effect, I have come up with an idea of ‘in vivo priming’, which means the hMSCs are primed directly on the failing hearts,” said Dr Ban.
Novel strategy: in vivo priming of hMSCs
To prove the concept, the research team loaded two types of MSCs into a tailor-made 3D-printed patch, namely the human bone marrow-derived MSCs, and the genetically engineered MSCs which have human hepatocyte growth factor protein. Hepatocyte growth factor (HGF) is involved in multiple biological activities, such as cell survival, blood vessel formation and anti-fibrotic activities, and important in adult organ regeneration and wound healing.
The patch, like a bandage, was then implanted on the top of the infarct area of the myocardial-infarction-induced heart of rats. “The genetically engineered MSCs can continuously secret human HGF protein to prime the hMSCs within the patch and make them ‘stronger’,” said Dr Ban.

Instead of directly injecting the genetically engineered cells into the heart, he added that encapsulating the cells in the patch for putting on the surface of the heart can help prevent mutation or other undesirable outcomes. And the patch is fabricated by 3D-printing of pig heart-derived Hepatocyte growth factor hydrogel, simulating the cardiac tissue-specific micro-environment.
It was found that the primed hMSCs had a higher survival rate compared with unprimed ones in the patches attached to the failing hearts. Those empowered hMSCs released greater amounts of paracrine factors beneficial for repairing damaged cardiac muscle tissues and regenerating vasculatures.
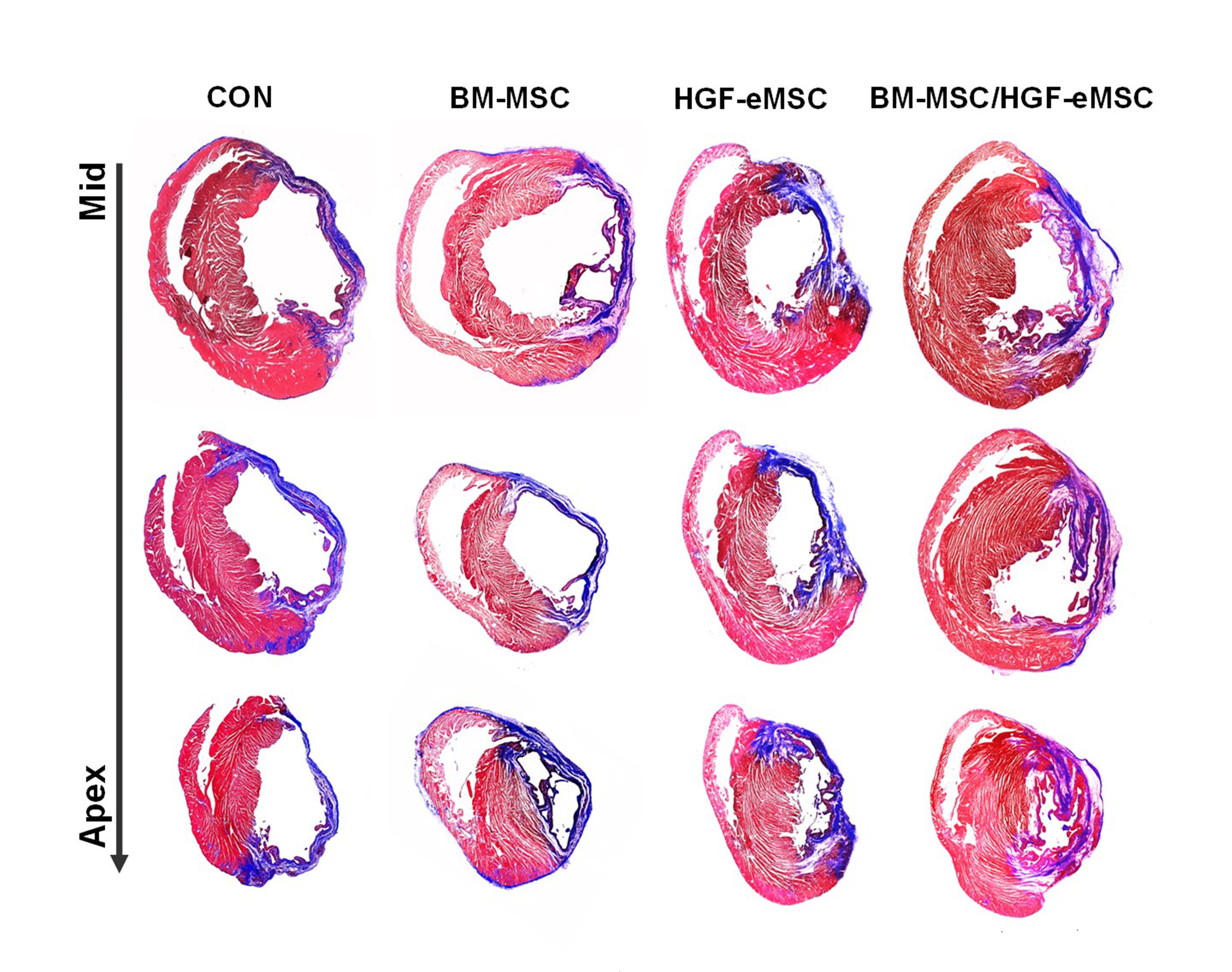
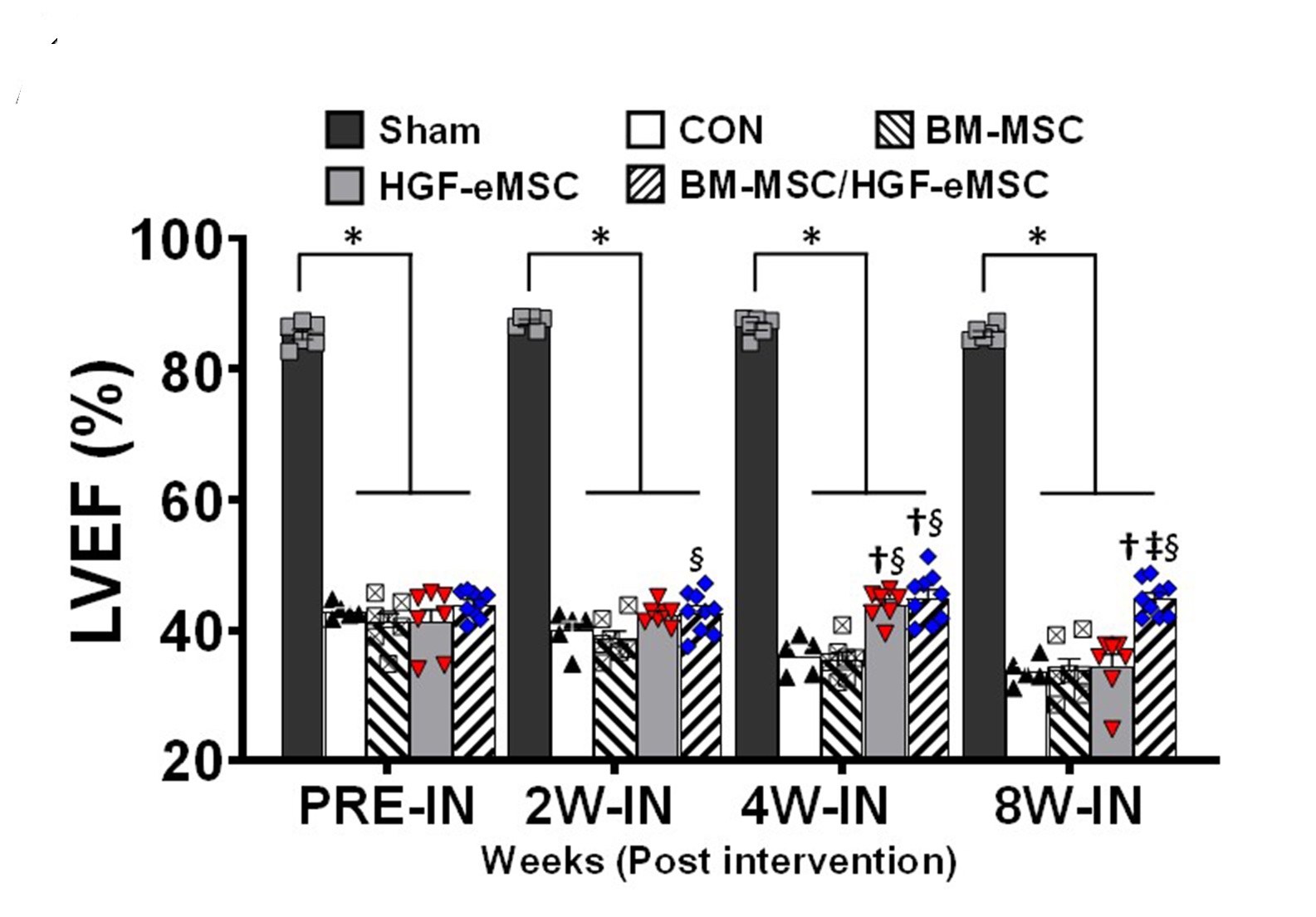
“We found that the primed cells can survive even after 8 weeks in the patch after implantation to the heart. Also, there is a significant improvement in cardiac function as well as vessel regeneration comparing to the unprimed cells,” said Dr Ban.
Great improvement of the priming effect
“Our team is the very first to achieve priming in hearts in vivo. But more importantly, by showing that in vivo priming of hMSCs can enhance the therapeutic potential for cardiac repair, we hope our study can bring significant implications for related stem cell therapy in future,” concluded Dr Ban. It took the team over two years to these achieve remarkable results. The team will explore the possibility of conducting the experiments on bigger animals and even clinical trials, as well as modifying the structure of the patch.
Dr Ban, Dr Jang Jinah from Pohang University of Science and Technology, as well as Professor Park Hun-Jun from The Catholic University of Korea are the leading authors of the paper and Mr. Lee Sunghun, a PhD student from Department of Biomedical Sciences at CityU also participated in this research.
The study was supported by CityU, Hong Kong Research Grants Council, National Research Foundation of Korea, Ministry of Education as well as the Ministry of Science and ICT in South Korea.
DOI number: 10.1126/sciadv.aay6994
Related Story: Novel dual stem cell therapy improving cardiac regeneration